Thalidomide-O-C6-NH2 hydrochloride
CAS No. 2245697-88-3
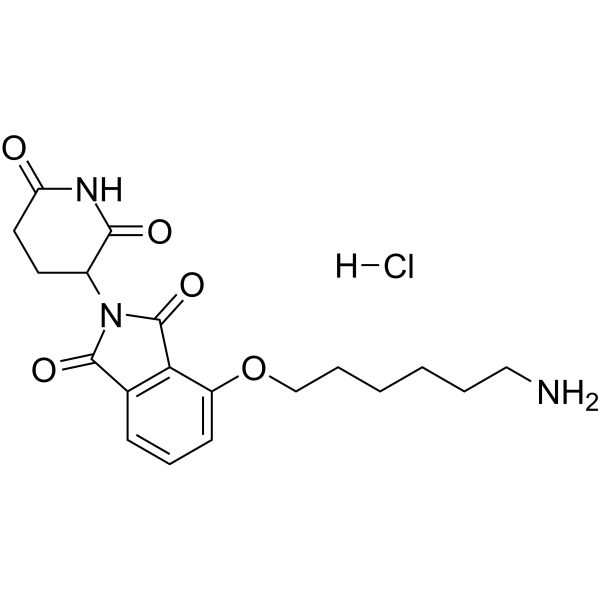
Thalidomide-O-C6-NH2 hydrochloride( 4-((6-aminohexyl)oxy)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride )
Catalog No. M28271 CAS No. 2245697-88-3
Thalidomide-O-C6-NH2 hydrochloride is a synthesized E3 ligase ligand-linker conjugate.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 35 | Get Quote |


|
| 10MG | 58 | Get Quote |


|
| 25MG | 110 | Get Quote |


|
| 50MG | 160 | Get Quote |


|
| 100MG | 241 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameThalidomide-O-C6-NH2 hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionThalidomide-O-C6-NH2 hydrochloride is a synthesized E3 ligase ligand-linker conjugate.
-
DescriptionThalidomide-O-C6-NH2 hydrochloride is a synthesized E3 ligase ligand-linker conjugate.
-
In Vitro——
-
In Vivo——
-
Synonyms4-((6-aminohexyl)oxy)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride
-
PathwayOthers
-
TargetOther Targets
-
RecptorNLRP3
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2245697-88-3
-
Formula Weight409.86
-
Molecular FormulaC19H24ClN3O5
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 83.33 mg/mL (203.31 mM)
-
SMILESO=C1N(C(CC2)C(NC2=O)=O)C(C3=C1C=CC=C3OCCCCCCN)=O.[H]Cl
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Gary Glick, et al. Substituted imidazo-quinolines as nlrp3 modulators. WO2018152396A1.
molnova catalog



related products
-
Etretinate
Etretinate is a RAR and RXR agonist previoulsy used to treat psoriasis. It induces brth defects and suppresses proliferation in cutaneous T-cell lymphoma models.
-
SP420
SP-420 is a novel orally active iron chelator.
-
D-Saccharic acid 1,4...
D-Saccharic acid 1,4-lactone hydrate is a potent β-glucuronidase inhibitor (IC50=48.4 μM). D-Saccharic acid 1,4-lactone hydrate possesses anticarcinogenic, detoxifying, and antioxidant properties.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


